Introduction
Bacterial infections have consistently posed a significant threat to humanity. For recipients of solid organ transplants, there are risks of donor-derived infections, intraoperative infections, and postoperative infections. For patients awaiting organ transplantation, if a bacterial infection is present at the intended transplant site, transplant centers often decline to perform the procedure, fearing that the transplanted organ may become infected. Antibiotic use has been effective in most instances, but as bacterial resistance has risen, cases of “superbug” infections, which can lead to graft failure and even threaten the recipient’s life, have become increasingly common. Bacteriophage therapy, a natural treatment unaffected by antibiotic resistance, has regained the attention of transplant physicians. Currently, clinical trials of bacteriophage therapy are underway in China, the United States, Europe, Israel, Georgia, and other countries; nearly 20 cases of bacteriophage therapy for transplant-related infections have been documented in published literature, and a clinical trial in Shanghai has treated 12 patients with organ transplant-related infections, 11 of which remain unpublished. Continue reading to learn more.
1. Bacteriophage Therapy
Bacteriophages are a class of microorganisms that specifically “consume” bacteria. They are widely distributed and abundant within the human body and the surrounding environment, playing a crucial role in maintaining bacterial balance. Lytic bacteriophages multiply and lyse bacteria after infection, possessing a natural ability for precise bacterial killing. Since the French-Canadian microbiologist Félix d’Hérelle pioneered bacteriophage therapy in 1919, it has spanned a century of history. During the early post-liberation period, China developed and produced bacteriophages, achieving notable success in preventing dysentery. In 1958, Professor Yu Yi from the Shanghai Institute of Immunology successfully treated burn patients infected with Pseudomonas aeruginosa using bacteriophages, marking a significant milestone in China’s microbiological field. However, since the industrial production and widespread use of antibiotics in the 1950s, bacteriophage therapy, with its specific bactericidal properties, has been gradually supplanted by broad-spectrum antibiotics. By the early 21st century, as bacterial resistance became an increasingly severe issue, bacteriophage therapy regained recognition and is now considered a highly promising next-generation antibacterial therapy.
2. Bacterial Resistance
The illustrious history of antibiotic use is paralleled by the history of bacterial resistance, which has become an increasingly severe problem. In 2009, the Infectious Diseases Society of America (IDSA) identified Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Escherichia coli (collectively known as ESKAPE) as the six “superbugs” posing the greatest clinical threat. In 2017, the World Health Organization (WHO) identified 12 “superbugs” in urgent need of new antimicrobial therapies, in addition to the six mentioned above. These bacteria exhibit significant drug resistance and a lack of effective antibiotics, making them primary targets for bacteriophage therapy.
3. Solid Organ Transplantation and Bacterial Infection
With advancements in clinical medicine, hygiene, and immunosuppressive drugs, organ transplantation and postoperative anti-rejection management have become increasingly sophisticated. Nevertheless, organ transplant recipients continue to face significant challenges from drug-resistant bacterial infections. Preoperative infections, perioperative nosocomial infections, and postoperative opportunistic infections due to immunosuppression are critical risk factors for graft failure and potentially life-threatening complications. The prevalence of multidrug-resistant bacteria among kidney, liver, heart, and lung transplant recipients reaches 14%, 15%, 25%, and 37%-51%, respectively. Additionally, refractory Pseudomonas aeruginosa and Mycobacterium abscessus infections frequently occur in patients with chronic lung disease awaiting lung transplantation.
In solid organ transplantation, there is a significant risk of infection with drug-resistant bacteria during organ harvesting, preservation, transportation, implantation, and wound healing. However, due to insufficient environmental bactericidal intensity, frequency, and efficacy, along with delays in bacterial identification and inadequate isolation measures, the spread of superbugs among hospitalized patients cannot be overlooked. Moreover, because transplant recipients require immunosuppressants to prevent organ rejection, opportunistic infections further elevate the risk of infection, often with greater severity.
4. Transplant Infection and Bacteriophage Therapy
To date, numerous published reports have documented bacteriophage therapy for transplant-related infections and its use in controlling bacterial infections in organ transplantation, involving a total of 19 patients (Table 1). The key details are as follows:
Patient categories: Include lung, kidney, liver, and heart transplants, as well as individuals awaiting lung transplantation, with the youngest being a 1-year-old child following a liver transplant.
Target pathogens: Include Pseudomonas aeruginosa, Klebsiella pneumoniae, Escherichia coli, Enterococcus faecium, Mycobacterium abscessus, Achromobacter, and Burkholderia cepacia.
Administration methods: Include intravenous injection, nebulized or bronchoscopic lavage (for lungs), local irrigation, gel or wet compress (for skin), nasogastric tube, and rectal suppository.
Combined antibiotic therapy: In most cases, bacteriophages were used alongside antibiotics (without altering the antibiotic regimen) for a duration ranging from a minimum of 5 days to a maximum of 3.5 years.
Treatment outcomes: Bacterial infections and clinical symptoms improved in 13 cases; target bacterial infections were controlled in 3 cases, enabling organ transplantation; target bacteria were eradicated in 2 cases, but clinical symptoms did not improve; neither target bacteria nor clinical symptoms improved in 1 patient.
Adverse reactions: Only one patient with a pulmonary infection developed a fever following bacteriophage therapy, potentially related to RNA phages.
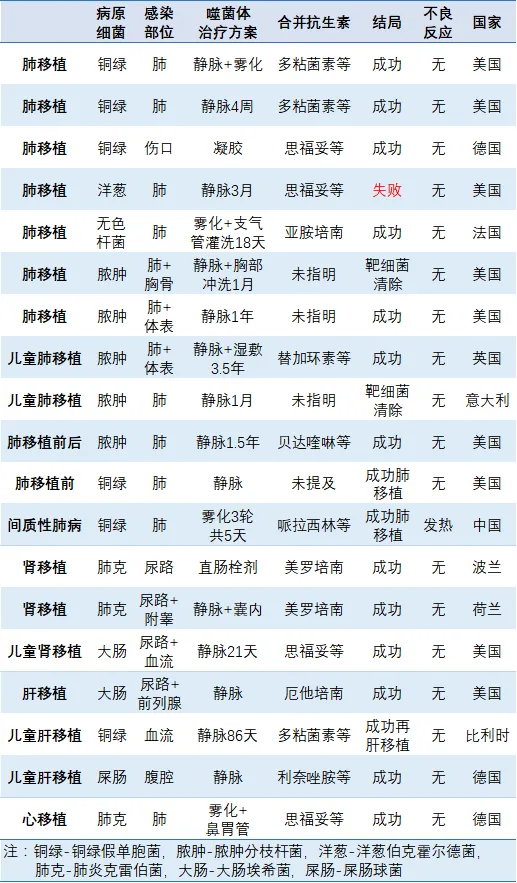
Table 1. Summary of Published Cases of Solid Organ Transplant-Related Infections (as of February 28, 2023)
5. Application Potential of Bacteriophages in Solid Organ Transplantation
Bacteriophages have been utilized to treat bacterial infections both before and after organ transplantation. Bacteriophage therapy has been implemented in China, including at Zhongshan Hospital Affiliated with Fudan University, Shanghai Public Health Clinical Center, Shanghai Jiahui International Hospital, Shenzhen Third People’s Hospital, and Shenzhen People’s Hospital. The Shanghai Bacteriophage and Drug Resistance Research Institute team has conducted bacteriophage therapy for more than 10 cases of transplant-related infections at Zhongshan Hospital and the Public Health Center, including 10 cases of infection following kidney transplantation, one case of infection after liver-kidney transplantation, and one case of interstitial pneumonia (leading to lung transplantation after bacteriophage therapy). These infections were caused by Klebsiella pneumoniae, Acinetobacter baumannii, and Pseudomonas aeruginosa. Administration methods included bladder irrigation (for urinary tract infections), aerosol inhalation (for pulmonary infections), catheter-based washing (for abdominal infections), and wet dressings (for wound infections). Treatment duration ranged from 1 to 5 rounds, with each round lasting 1–3 days and involving two daily bacteriophage administrations. Compared to conventional antibiotic therapy, personalized bacteriophage screening for drug-resistant bacteria in patients often yields better therapeutic outcomes and fewer side effects.
Drug-resistant pathogens may also be present in hospitals, operating rooms, and home environments, contributing to transplant-related infections. Conventional chemical (e.g., chlorine-based disinfectants) or physical (e.g., ultraviolet) disinfection methods are often unsuitable for environments containing people, valuables, or equipment due to their potential harm to humans and corrosive effects on objects. As a natural bactericide that specifically targets bacteria (without affecting human cells), bacteriophages can effectively eliminate drug-resistant bacteria in these settings. The Shanghai Bacteriophage and Drug Resistance Research Institute team has achieved promising results in preventing and controlling nosocomial infections using bacteriophage environmental sprays in Taiwan and Shanghai.
Additionally, during organ preservation and transportation, perfusion fluids may become contaminated with drug-resistant bacteria, leading to donor-derived infections. Although no clinical trials have been reported, there is potential to prevent superbug infections in organs during preservation and transportation by incorporating a broad-spectrum bacteriophage cocktail targeting common clinical pathogens into the organ perfusion fluid. Bacteriophages can also be employed to sterilize donor organs infected with identified pathogens, preventing the wastage of donor organs.
Summary
Organ transplantation is the preferred treatment for end-stage organ failure. Multidrug-resistant bacterial infections significantly impact the success rate of organ transplantation and the post-transplantation health of recipients. For many years, the mechanisms of action and potential risks of bacteriophage therapy have remained inadequately understood within the academic community, making its development a focal point of interest. Additionally, bacteriophages’ high host specificity (narrow bactericidal spectrum), the propensity for bacteria to develop phage-resistant mutations, and other limiting factors have hindered the efficacy of bacteriophage therapy, preventing its widespread clinical adoption amid the dominance of broad-spectrum antibiotics. Bacteriophage therapy remains challenging to implement, with a lack of standardized, large-scale, controlled clinical trials. No bacteriophage drugs have been approved in China, the United States, or the European Union; both the cases cited above and ongoing bacteriophage therapies are part of clinical trials. With rapid advancements in molecular biology, synthetic biology, and sequencing technologies, multicenter bacteriophage therapy applications—such as those at Shanghai Zhongshan Hospital, Shanghai Public Health Clinical Center, Shanghai Jiahui International Hospital, Shenzhen Third People’s Hospital, Shenzhen People’s Hospital, and the First Affiliated Hospital of Xi’an Jiaotong University—along with the emergence of companies specializing in innovative bacteriophage drugs and personalized medicine technologies (e.g., ChemTime, Target Antibiotics, and SEATO BIO), bacteriophages are poised for expansive growth in preventing, controlling, and treating transplant-related drug-resistant bacterial infections.
References:
[1] Wu Nannan, Zhu Tongyu. Application of Bacteriophages in Solid Organ Transplantation. Organ Transplantation, 2019, 10(4): 410-415.
[2] Nicholls and Aslam. Role of Bacteriophage Therapy for Resistant Infections in Transplant Recipients. Curr Opin Organ Transplant. 2022 Dec 1;27(6):546-553.
[3] Uyttebroek et al. Safety and Efficacy of Phage Therapy in Difficult-to-Treat Infections: A Systematic Review. Lancet Infect Dis. 2022 Aug;22(8):e208-e220.