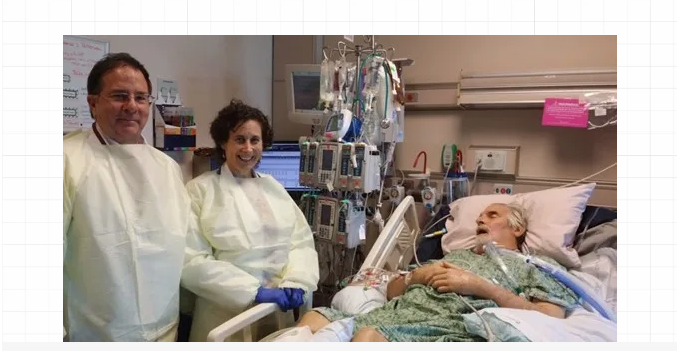
图:“金字塔”案例患者Tom Patterson病床前的Schooley(左一)
导语
2024第七届靶向噬菌体治疗大会参会人员张三在《2024马耳他噬菌体治疗大会见闻(上)》中放言对一些报告详细介绍。可是最近忙于各种安利噬菌体新品(霏津四合一噬菌体制剂),第二更姗姗来迟。不过终于来了。本篇介绍本次会议唯一的主旨报告,来自加州大学圣地亚哥分校的Robert T. Schooley教授。这位大牛是Clinical Infectious Diseases杂志前主编(2017-2022年),美国过敏和传染病研究所(NIAID)艾滋病临床试验小组(ACTG)前组长(1995-2002)。他还时常署名“Chip” Schooley高谈阔论噬菌体治疗,谁叫他是美国噬菌体治疗重启标志性事件“金字塔案例”的主治医生和加州大学圣地亚哥分校创新噬菌体应用与治疗中心(IPATH)联合主任呢。这次马耳他大会上,“Chip”说了啥呢?
“Chip” Schooley教授的报告题目是Clinical Trials in Phage Therapeutics: Looking Under the Hood(蹲在安全柜下面看噬菌体治疗临床试验)。由于Schooley在会议前夕感染疱疹病毒(没听错的话)尚未痊愈,因此取消了线下行程,改为线上录播。张三错失机会问Schooley他的别名“Chip”是哪个意思。
Schooley是美国噬菌体治疗复苏的关键人物之一,且是其中的医生角色,曾在数个顶级杂志安利噬菌体治疗,如2020年发表于Nature Microbiology的“俺看噬菌体就是活的抗生素”(Treat phage like living antibiotics);2022年发表于Annual Review of Medicine的“耐药菌感染俺选噬菌体治疗”(Phage Therapy for Antibiotic-Resistant Bacterial Infections);2023年发表于Cell的“噬菌体治疗:从理论到未来实践”(Phage therapy: From biological mechanisms to future directions)。Schooley还是美国FDA噬菌体治疗相关咨询的座上宾和葛兰素史克、艾伯维、LyseNTech等公司的顾问,因此对美国噬菌体治疗审批十分了解。
在报告开篇,Schooley首先介绍了美国噬菌体治疗的监管路径。由于美国尚未有审批上市的噬菌体药品,因此噬菌体治疗仍是实验性的、必须在FDA生物制品评价与研究中心(CBER)疫苗研究和审查办公室的监督下进行管理。个案患者可以在FDA研究型新药(IND)的研究者发起的临床试验(IIT)监管框架下进行噬菌体治疗,即常说的同情心治疗路径。需要通过该网站联系FDA并递交资料https://www.fda.gov/about-fda/center-drug-evaluation-and-research-cder/physician-request-single-patient-ind-compassionate-or-emergency-use。
同情心伦理通常需要先获得医院的机构审查委员会(IRB)审查通过,再申请FDA审批。FDA的科学家要求医生提供有关病例的信息、将要使用的噬菌体制剂以及治疗计划。对于需要紧急治疗的患者,还可走紧急eIND路径,电话联系FDA,最快可在一天内审批通过。在这种情况下,患者(或授权代表)的知情同意书还是必须提供的,但不需要医院机构审查委员会的审查,只需在治疗开始后5天内通知医院的机构审查委员会。
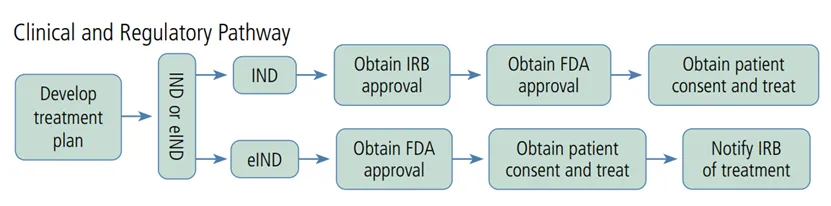
图:截自Schooley在Topics in Antiviral Medicine发表的综述论文(https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10089292/pdf/tam-31-23.pdf)。
注:IND试验性新药;eIND紧急使用的试验性新药,IRB机构审查委员会,FDA美国食药监局。
然后,Schooley就抛出他被问到许多次的灵魂拷问:噬菌体治疗欣欣向荣,为何还要做临床试验?虽然Schooley是坚定的挺噬菌体治疗派,但作为临床医生,Schooley也坚定认为仍需要开展高质量的噬菌体治疗临床试验,理由有三:
1. 虽然许多人说已有的临床数据已经充分证明了噬菌体治疗的有效性,临床试验没有必要,俺认为临床试验的目的并不是为了某种药物获得批准,而是学习如何最好的使用它。
2. 虽然过去五六年学院派的噬菌体治疗尝试越来越成功,但只有商业化才是噬菌体治疗规模化应用到患者群体的唯一路径。
3. 不管是商业公司去研究噬菌体药物还是医师临床使用噬菌体治疗,都需要在与抗生素相同的评价指标下积累噬菌体安全性和有效性的数据。
那么,如何利用正在进行的和接下来的临床试验来更好的优化噬菌体治疗呢?
Schooley从以下几点给出建议:
噬菌体配型:需要标准化,开始治疗后也需要监测细菌对噬菌体的敏感性;
药代动力学和药效学:如何评价给药路径、剂量和间隔才是最佳的?(即:给药方案是否有效的将噬菌体给到感染部位,且其在感染部位的裂解活性是否能够坚持到感染解决或人体免疫能够应付?)如何评价抗生素的影响?
(针对噬菌体的)天然免疫和获得性免疫:重要吗?什么情况下重要?如果重要,如何减轻它的影响?
提高噬菌体活性:(外部)噬菌体-噬菌体、噬菌体-抗生素、噬菌体-人体免疫协同作用;(内部)工程噬菌体(爆发量、裂解力、宿主谱、水解生物膜能力、对抗噬菌体抗性相关的改造)。
Schooley的观点,您认同吗?欢迎留言讨论。张三在本次大会的会场上也听到有不同意见。参加学术会议最有趣的便是这些观点的碰撞。比如,那位来自德国Brenner律师事务所的医学法和人权法专家Barbara Brenner。这不,接下来张三最最期待的便是8月14日至18日将在上海举办的2024第七届超级细菌感染与噬菌体防治前沿论坛。去年500人盛况历历在目,本届聚焦噬菌体临床应用和未来技术。除了精彩纷呈的主论坛报告,还特设种子培训营和90年史料展。张三期待在会上见到您,一起追忆历史,播种未来!
👇👇👇👇

-300x169.png)

