October 28, 2024, Immune Tolerance


The 2018 Nobel Prize in Physiology or Medicine was announced on October 1, a day of national celebration. One of the recipients was James Allison, Professor and Chair of the Immunology Department at the University of Texas MD Anderson Cancer Center. Professor Allison discovered that a protein called CTLA-4 on the surface of immune cells acts as a “molecular brake,” halting the immune response. Inhibiting CTLA-4 triggers T-cell proliferation and attack on tumor cells, earning him the Nobel Prize.
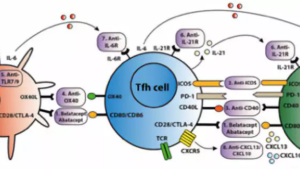
Indeed, in the field of organ transplantation, there is a novel immunosuppressant closely related to the CTLA-4 molecule. It was approved by the U.S. Food and Drug Administration (FDA) in 2011 to prevent acute rejection in adult kidney transplant patients. It is called belatacept (Nulojix).
Belatacept is a selective T-cell costimulatory signal blocker, comprising CTLA-4 fused to the Fc domain of a human immunoglobulin antibody. It effectively prevents the binding of CD28 to B7 molecules, leading to the development of a novel anti-CD28 antibody. It acts as a “molecular brake,” inhibiting T-cell activation and protecting the transplanted organ from rejection. Furthermore, it does not suppress the body’s immune response to other viruses (e.g., BK virus) or pathogens, thus reducing the risk of infection post-kidney transplantation compared to other immunosuppressants.
As a novel immunosuppressant, belatacept is similar to cyclosporine but surpasses it in preserving long-term graft kidney function, possibly due to its lower nephrotoxicity. Two multicenter, randomized, controlled clinical studies assessed the efficacy and safety of belatacept in kidney transplant patients. In the belatacept and cyclosporine groups, and in the control group, the acute rejection rates were 24.0% and 22.8%, respectively, and the graft loss rates were 2.2% and 3.6%, respectively. The glomerular filtration rate at 1 year was significantly higher in the belatacept group than in the cyclosporine group. The 5-year and 7-year follow-up results of the BENEFIT-EXT trial also confirmed better allograft survival and sustained improvement in kidney function with belatacept compared to cyclosporine.
However, belatacept is not without flaws. First, it is associated with some serious adverse reactions, such as lymphoproliferative disorders and progressive multifocal leukoencephalopathy following organ transplantation. Second, belatacept is currently being evaluated as an alternative immunosuppressant, but large-scale, long-term follow-up data are lacking, and it is not yet available in the Chinese market, so domestic kidney transplant patients have not yet had the opportunity to use it.
Written by | Jina Wang, Edited by | Jina Wang, Photography | G.T.
This article is an original publication of the “Kidney Transplantation, Zhongshan Hospital, Fudan University” WeChat public account. Reproduction requires authorization from this account and the original author, with proper attribution. To care for your kidneys, begin by following the “Kidney Transplantation, Zhongshan Hospital, Fudan University” WeChat public account. You can also click [Read the Original] to explore other articles in this issue.